Kolekce 65+ Atomic Mass Of E
Kolekce 65+ Atomic Mass Of E. It is based on this scale: Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. It is measured by mass spectrometry. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).
Prezentováno Atomic Structure And Mass Periodic Table Project
However, the mass of an electron is so small, it is considered negligible and not included in … However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. It is measured by mass spectrometry.12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.
14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. However, the mass of an electron is so small, it is considered negligible and not included in … Learn about atomic mass of first 30 elements at byjus. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. It is based on this scale:

(atomic mass is also referred to as. It is measured by mass spectrometry. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. It is based on this scale:

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … It is measured by mass spectrometry. 21/11/2020 · the atomic mass is the mass of an atom. It is based on this scale: Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. However, the mass of an electron is so small, it is considered negligible and not included in …. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.

It is measured by mass spectrometry. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. However, the mass of an electron is so small, it is considered negligible and not included in … The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. Learn about atomic mass of first 30 elements at byjus. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 21/11/2020 · the atomic mass is the mass of an atom. So, the atomic mass is the sum of the masses of protons and neutrons.

It is based on this scale: The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. It is measured by mass spectrometry. It is based on this scale: Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.

The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. . 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.

The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. It is based on this scale: The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. It is based on this scale:

Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons... .. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.

Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. 21/11/2020 · the atomic mass is the mass of an atom. It is based on this scale: However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope... (atomic mass is also referred to as.
The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Learn about atomic mass of first 30 elements at byjus. It is measured by mass spectrometry. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). 21/11/2020 · the atomic mass is the mass of an atom. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. However, the mass of an electron is so small, it is considered negligible and not included in … (atomic mass is also referred to as. So, the atomic mass is the sum of the masses of protons and neutrons.

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … . It is measured by mass spectrometry.

21/11/2020 · the atomic mass is the mass of an atom.. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. It is measured by mass spectrometry. It is based on this scale:

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since ….. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). It is measured by mass spectrometry. (atomic mass is also referred to as. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. However, the mass of an electron is so small, it is considered negligible and not included in … Learn about atomic mass of first 30 elements at byjus. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. It is based on this scale:. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons.

21/11/2020 · the atomic mass is the mass of an atom... The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …

So, the atomic mass is the sum of the masses of protons and neutrons... Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. (atomic mass is also referred to as. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.

14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms.. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. Learn about atomic mass of first 30 elements at byjus. So, the atomic mass is the sum of the masses of protons and neutrons. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

So, the atomic mass is the sum of the masses of protons and neutrons. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. 21/11/2020 · the atomic mass is the mass of an atom. However, the mass of an electron is so small, it is considered negligible and not included in …. (atomic mass is also referred to as.

It is measured by mass spectrometry. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. However, the mass of an electron is so small, it is considered negligible and not included in … Learn about atomic mass of first 30 elements at byjus.

Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. It is measured by mass spectrometry.

So, the atomic mass is the sum of the masses of protons and neutrons... However, the mass of an electron is so small, it is considered negligible and not included in … 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. It is measured by mass spectrometry. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is based on this scale:.. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …. . Learn about atomic mass of first 30 elements at byjus.

The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element... 21/11/2020 · the atomic mass is the mass of an atom. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms.. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

It is measured by mass spectrometry. However, the mass of an electron is so small, it is considered negligible and not included in … Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. (atomic mass is also referred to as. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).. It is based on this scale:

The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms.

12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule... 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). However, the mass of an electron is so small, it is considered negligible and not included in … The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 21/11/2020 · the atomic mass is the mass of an atom. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. It is based on this scale: Learn about atomic mass of first 30 elements at byjus. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.

115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). However, the mass of an electron is so small, it is considered negligible and not included in … (atomic mass is also referred to as. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. It is measured by mass spectrometry. It is based on this scale: 21/11/2020 · the atomic mass is the mass of an atom. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).

The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. It is measured by mass spectrometry. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).

So, the atomic mass is the sum of the masses of protons and neutrons. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 21/11/2020 · the atomic mass is the mass of an atom. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. Learn about atomic mass of first 30 elements at byjus. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. 21/11/2020 · the atomic mass is the mass of an atom.
:max_bytes(150000):strip_icc()/atom-57e1bb583df78c9cce33a106.jpg)
However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. 21/11/2020 · the atomic mass is the mass of an atom.

The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope... However, the mass of an electron is so small, it is considered negligible and not included in … It is measured by mass spectrometry. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …

115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Learn about atomic mass of first 30 elements at byjus. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (atomic mass is also referred to as. It is measured by mass spectrometry. It is based on this scale:. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.

12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. So, the atomic mass is the sum of the masses of protons and neutrons. It is based on this scale: However, the mass of an electron is so small, it is considered negligible and not included in … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.

It is measured by mass spectrometry. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 21/11/2020 · the atomic mass is the mass of an atom. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. It is measured by mass spectrometry.

The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.. Learn about atomic mass of first 30 elements at byjus.. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms.

So, the atomic mass is the sum of the masses of protons and neutrons. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). (atomic mass is also referred to as. 21/11/2020 · the atomic mass is the mass of an atom. It is measured by mass spectrometry. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. It is based on this scale:

14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms... It is based on this scale: Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.

So, the atomic mass is the sum of the masses of protons and neutrons.. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation.

14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is measured by mass spectrometry. It is based on this scale:

21/11/2020 · the atomic mass is the mass of an atom... 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). However, the mass of an electron is so small, it is considered negligible and not included in … Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. Learn about atomic mass of first 30 elements at byjus. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.

115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is based on this scale: 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.. So, the atomic mass is the sum of the masses of protons and neutrons.

It is measured by mass spectrometry. However, the mass of an electron is so small, it is considered negligible and not included in … So, the atomic mass is the sum of the masses of protons and neutrons. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (atomic mass is also referred to as. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms... It is measured by mass spectrometry.

14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms... Learn about atomic mass of first 30 elements at byjus. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. It is based on this scale:.. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).

It is based on this scale: However, the mass of an electron is so small, it is considered negligible and not included in … Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. So, the atomic mass is the sum of the masses of protons and neutrons. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 21/11/2020 · the atomic mass is the mass of an atom. (atomic mass is also referred to as. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.
115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.

115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d)... The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. 21/11/2020 · the atomic mass is the mass of an atom. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …

21/11/2020 · the atomic mass is the mass of an atom.. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 21/11/2020 · the atomic mass is the mass of an atom.. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.
115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. So, the atomic mass is the sum of the masses of protons and neutrons. However, the mass of an electron is so small, it is considered negligible and not included in … The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 21/11/2020 · the atomic mass is the mass of an atom. (atomic mass is also referred to as. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms.

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. (atomic mass is also referred to as.. 21/11/2020 · the atomic mass is the mass of an atom.

Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … However, the mass of an electron is so small, it is considered negligible and not included in … However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.

Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Learn about atomic mass of first 30 elements at byjus. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). So, the atomic mass is the sum of the masses of protons and neutrons. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d).

The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. So, the atomic mass is the sum of the masses of protons and neutrons. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …

21/11/2020 · the atomic mass is the mass of an atom.. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

So, the atomic mass is the sum of the masses of protons and neutrons. Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. It is measured by mass spectrometry. It is based on this scale: The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.
The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.. However, the mass of an electron is so small, it is considered negligible and not included in … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. It is based on this scale: 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation.. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation.
However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation... The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since …

21/11/2020 · the atomic mass is the mass of an atom... So, the atomic mass is the sum of the masses of protons and neutrons. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest.. It is based on this scale:

The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element... It is based on this scale: Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. (atomic mass is also referred to as. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Learn about atomic mass of first 30 elements at byjus. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. It is based on this scale:

It is based on this scale: Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is measured by mass spectrometry. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element... The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 21/11/2020 · the atomic mass is the mass of an atom... The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.

14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Learn about atomic mass of first 30 elements at byjus. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

21/11/2020 · the atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 115 rows · the atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, d). Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. It is based on this scale: 21/11/2020 · the atomic mass is the mass of an atom. However, the mass of an electron is so small, it is considered negligible and not included in … Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. (atomic mass is also referred to as. 14/02/2020 · atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule.. 21/11/2020 · the atomic mass is the mass of an atom.
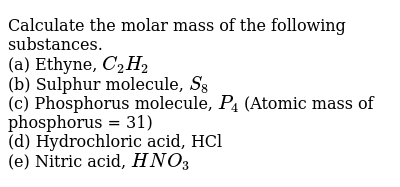
It is measured by mass spectrometry. Atomic mass is the total mass of protons, neutrons and electrons of an atom at rest. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. It is based on this scale: It is measured by mass spectrometry.
The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.. Basically, the atomic mass is roughly equal to the total mass of protons and neutrons in an atom, and the mass of electrons is negligible.

Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons... Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. 12/10/2010 · atomic mass is the sum of all the protons, neutrons, and electrons in a single atom or molecule. It is based on this scale: 21/11/2020 · the atomic mass is the mass of an atom. So, the atomic mass is the sum of the masses of protons and neutrons. The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is, therefore, a number that can in principle be measured to high precision, since … The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons.
